TAM Global Study Challenges Stem Cell Claims for Wharton's Jelly Commercial Products
PR Newswire
NASHVILLE, Tenn., Jan. 29, 2026
NASHVILLE, Tenn., Jan. 29, 2026 /PRNewswire/ -- TAM Global announced today the publication of a peer-reviewed study demonstrating that commercially available Wharton's jelly products do not contain clinically meaningful levels of viable stem cells. The article, "Critical Evaluation of Compositions and Clinical Relevance of Wharton's Jelly-Derived Biologics," published in the Journal of Translational Medicine, concludes these products should not be represented or assumed to function as stem cell treatments.
Conducted by scientists at TAM Global and the Center for Scientific Research and Higher Education at Ensenada, the review exposes the gap between marketing claims and biological reality in the regenerative medicine marketplace. The authors distinguish Wharton's jelly products, which contain negligible stem cells, from true mesenchymal stem cell therapies, warning that the two types of products are frequently conflated in commercial messaging.
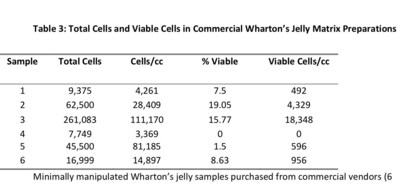
Wharton's jelly products are commercially sold as injectable or implantable biologic materials derived from donated umbilical cords that are minimally processed and marketed for various tissue healing applications. TAM's publication is directed against marketed Wharton's jelly products being described using stem cell-related language, which can lead to misconceptions that they contain living stem cells. The authors demonstrate that, in practice, commercially available Wharton's jelly products contain little to no viable stem cells.
"There is a fundamental difference between Wharton's jelly products and stem cell therapy," said Ed Clay, executive leader at TAM Global and the Cellular Performance Institute and coauthor of the study. "Our analysis shows that while minimally manipulated Wharton's jelly products function as structures to support tissue repair, they do not deliver functional stem cells in clinically meaningful doses. This paper helps reset the conversation so the field can mature responsibly, with realistic claims, better clinical alignment, and stronger long-term credibility"
"Calling a Wharton's jelly product a stem cell therapy is like calling Fanta Orange juice. It sets everyone up for disappointment, patients and clinicians alike," said Francesco Marincola, Chief Scientific Officer of TAM Global and co-author of the study. "If the mechanism of action of Wharton's jelly is a structural support or biochemical signaling scaffold, that's a legitimate biologic strategy. But it must be represented honestly and evaluated on its own merits."
The authors emphasize that the review is not intended to dismiss Wharton's jelly–derived biologics, but to correct the record. By clearly separating ECM-based products from true stem cell therapies, which require fresh tissue processing, targeted cell isolation, and laboratory expansion to reach therapeutic cell numbers, the paper urges greater transparency, more responsible marketing, and better-informed clinical decisions as the regenerative medicine field continues to mature.
View the full publication in the Journal of Translational Medicine here
About TAM Global
TAM Global is a leading translational science company dedicated to developing evidence-based cell therapies built on rigorous biology, standardized characterization, and validated clinical outcomes. With research operations in Boston, Nashville and Tijuana, TAM Global is a multidisciplinary team of 16 PhD scientists and 42 Medical doctors with a decade-long commitment to advancing regenerative medicine and cancer therapeutics. TAM Global is setting new benchmarks for safety, reproducibility, and scientific integrity in the global cell therapy landscape.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/tam-global-study-challenges-stem-cell-claims-for-whartons-jelly-commercial-products-302674253.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/tam-global-study-challenges-stem-cell-claims-for-whartons-jelly-commercial-products-302674253.html
SOURCE The TAM Center